The definition of “medical device” is defined in Article 2 of the Pharmaceutical and Medical Equipment Law (Law concerning the Assurance of Quality, Efficacy, and Safety of Drugs and Medical Devices, etc.).
What are the “medical devices”?
①To be used for the diagnosis, treatment, or prevention of disease in humans or animals.
②Medical instruments, etc. that are intended to affect the structure or function of the body of a person or animal
③It is stipulated by a Cabinet Order.
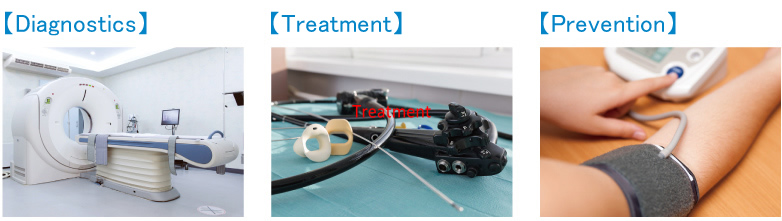
Also, in ①, the term “medical equipment etc.” is used, but the definition of “medical equipment etc.” is clearly defined in the definition of drugs under the Pharmaceutical Affairs Law.
“Medical instruments, etc.” refers to machine instruments, dental materials, medical supplies, satellite supplies, programs and recording media that record them.
There are some ambiguous definitions of medical devices based solely on ① and ② above, and it is difficult to clearly distinguish them from health equipment and general equipment. For example, those that build muscles or adjust weights, such as bench presses and running machines installed in sports clubs, are not considered medical devices. However, chair-type massage chairs are considered as medical equipment.
Therefore, as shown in ③ above, the classification and name of medical devices are clearly specified by the list in Appendix 1 of the Enforcement Order of the Pharmaceutical Affairs Law (Enforcement Order for Ensuring Quality, Effectiveness and Safety of Drugs and Medical Devices, etc.). In other words, the machinery and equipment listed in Appendix 1 of the Enforcement Order of the Pharmaceutical Affairs Low are defined as medical devices, and they are classified into 84 types. (As of March 2016)
















